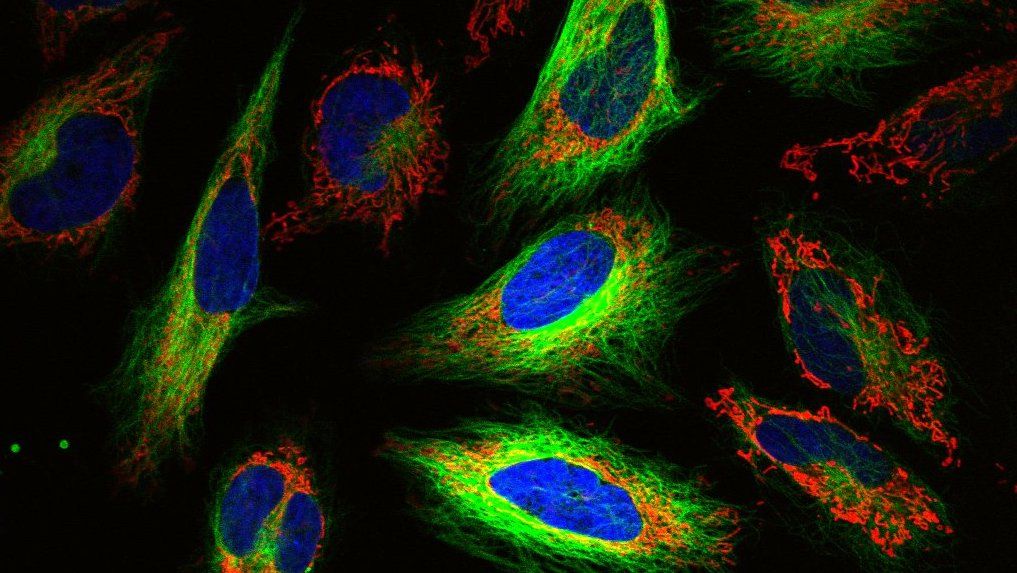
If you’ve ever delved into the world of biotechnology and drug discovery, you may have come across the term high-content screening, or its cousins high-content imaging and high-content analysis. But what exactly does “high-content” mean? How are these concepts different from each other? And how does the drug discovery pipeline benefit from “high-content” techniques?
Contents:
- What is High-Content Screening (HCS)?
- Why is it used?
- What is screened?
- What models are used?
- High-Content Screening in practice
- Instruments and Solutions for HCS
- Read more about HCS
- References
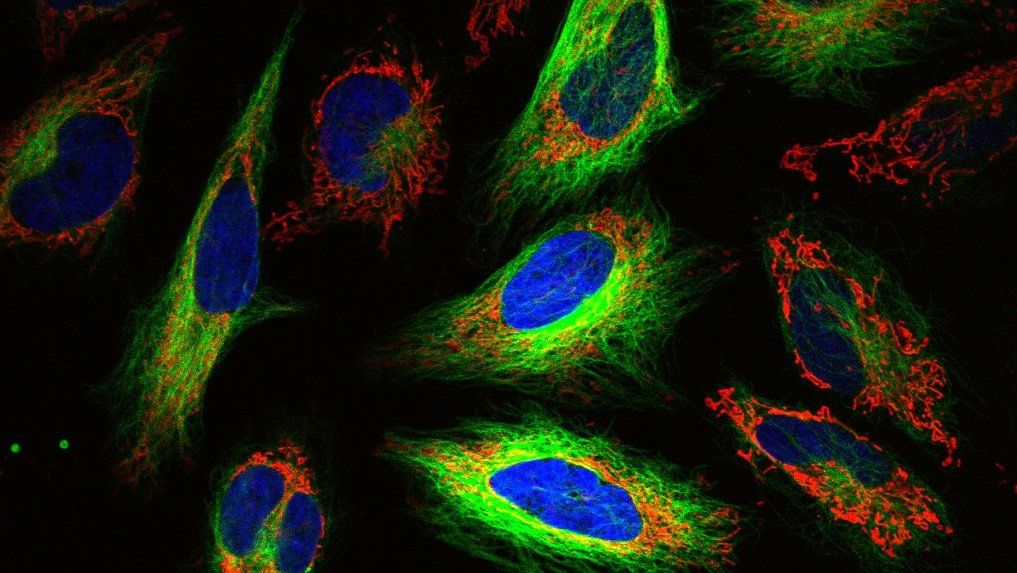
What is High-Content Screening (HCS)?
You have probably heard of High-throughput screening, in which a large number of drugs (or other compounds) are tested in parallel to find the one that gives some desired result. The term High-Content Screening is similar, but specifically refers to a process where, instead of just one endpoint, you measure many different endpoints or outputs.
High-Content Imaging (HCI) specifically refers to a version of HCS that uses imaging, or microscopy, to generate data. Given that microscopy has evolved to the point where you can incorporate a litany of tools, and is used quite a bit in High-Content Screening, these two terms are sometimes used interchangeably.
Finally, you might hear about High-Content Analysis (HCA). An HCS experiment, given that it involves many measurements made on many different testing conditions, is going to generate a high volume of data. HCA simply refers to the methods used to analyze the masses of data produced by High-Content Screening.
When someone says “High-Content Screening” it might evoke images of multi-well plates, automated pipetting systems, and batched image processing. But HCS is a broad term that can apply to assays involving a wide range of model systems, testing paradigms, and experimental outputs. The exact nature of a high-content screen needs to be designed carefully according to the goals of the experiment.
Why is it used?
A screen is used to sift through a large number of candidates, from drugs to genes, to find the one that is useful or interesting. In the world of therapeutics, screens are a powerful early-stage discovery tool. Researchers can get their hands on huge sets of different compounds--some of which might already be used as therapeutics--and test them on a particular model of a disease. If a compound shows positive results in the initial screen, it can be followed up with more in-depth testing.
In the past, screens were limited in the amount of answers they could provide. Running hundreds, thousands, or even more experiments in parallel was (and still is) a huge logistical challenge. The assays researchers use have to be consistent and reproducible, and there has to be a way to take accurate measurements each and every time. To run a successful screen, even with only one output, is a big endeavor.
Over time, technological advances have expanded the biologist’s toolbox. There are more options for assays to be run, more accurate models to be tested, and more powerful software for handling data--researchers are not as limited in the amount of data they can generate and analyze in a screen. Hence, the move towards multiple endpoints, and the rise in popularity of High-Content Screening.
Imaging is a perfect tool for analyzing multiple outputs at once, and so has become a primary component of HCS. A single micrograph can contain multitudes of information about a cell--its morphology, intracellular goings-on, interactions with other cells and proteins in its environment, and so on. And with technological improvements in imaging tools--from the dyes, stains, and constructs used to visualize cells, to the microscopes themselves--researches can more easily and consistently image large numbers of samples at once. An image can also provide clues to things that you may not have even been looking for in the first place, like off-target effects.
High-Content Imaging combines the ability of biologists to test huge numbers of compounds and models in parallel with the ability of a microscope to generate huge amounts of data. This has made it an incredibly powerful tool, and a foundational method that can be applied to multiple steps of the drug discovery pipeline.
What is screened?
High-Content Screening is defined by the output (specifically outputs, plural) of a screen, and not the input. Therefore, screens on all types of compounds and treatments can theoretically fall under the “HCS” umbrella.
As mentioned before, a common use of HCS is to screen small molecule compounds, or drugs, for potential therapeutic effects. In addition to small molecules, therapeutics can also be large macromolecules, such as antibodies.
Besides drug discovery, HCS can be applied to the target discovery step of the pharmaceutical development process. In this step, researchers attempt to find which genes or proteins are involved in a particular biological process. Once the important gene or protein is found, they can then work to develop therapeutics that target that specific component.
Traditionally, RNAi screens have been used for target discovery as they allowed for a large number of genes--even the entire genome--to be probed (albeit indirectly) for an effect. New genome editing techniques, most notably CRISPR-Cas9 genome editing, are unlocking even more opportunities for High-Content Screening applications.
What models are used?
As biotechnology has become more advanced, so have the models available to researchers.To date, many large-scale experiments have been performed on immortalized cell lines. These cells might not always be an ideal model of human biology, but are easier to consistently culture and test in large numbers. Indeed, getting enough samples to test is still one of the biggest obstacles in HCS today.
Despite the challenges, researchers are working to apply HCS to more sophisticated models. Induced pluripotent stem cells (iPSCs) are a promising candidate for HCS, as they allow researchers to test new and different cell types, and to make models that are specific to a certain person or group of people with a disease. However, iPSCs suffer from the problem of scale--they are expensive and time-consuming to make, especially in the numbers needed for a screen.
Beyond simple cell lines, multicellular structures such as organoids have captured the interest of researchers for their ability to more accurately model how cells behave in the human body. It’s unsurprising that in recent years, methods have been developed to use organoids for high-content screens. However, the use of organoids for screens comes with even more challenges: in addition to being difficult to make in large numbers, organoids can be both inconsistent and heterogeneous. Finally, organoids are three-dimensional, and adding a whole new axis to a high-content imaging experiment exponentially increases the amount of data that needs to be acquired and processed.
Finally, it’s not just cells and multicellular structures in a dish that can be screened. High-content screening has been applied to whole organisms, including fruit flies and zebrafish larvae, for the purpose of testing how compounds affect an entire in vivo physiological system.
High-Content Screening in practice
Even though High-Content Screening refers to a broad category of screening systems and experiments, there are some commonly used tools and techniques across High-Content screens. According to 2016 survey [3]: most respondents who were experienced in HCS used immortal cell lines and primary cells in 2D culture, measured around 6-10 outcomes, and typically screened for phenotypes using fluorescent dyes, expressed proteins, and commercial antibodies. Some commonly measured outcomes include cell counts, signal expression changes, morphology changes, and movements of specific proteins or structures.
In a classic example of the potential for High-Content methods to benefit drug discovery, Garripa et al. used High-Content Imaging to find compounds that affect the activity of G-Coupled Protein Receptors (GPCRs) using fluorescently tagged proteins. [4] Many of these GPCRs have unknown functions, and the ones that are better understood can have profound effects on human physiology. Being able to simultaneously learn what these proteins do and what substances affect them can have profound implications for human health.
In an advancement for our basic understanding of the brain, a High-Content Imaging approach was used to find out which genes affect the growth of brain cells. [5] Neurons don’t continue to grow once they’re mature, for reasons we don’t completely understand. Blackmore et al. were able to leverage High-Content techniques to test a large number of genes, using neuron growth as their output, and find the ones that either improved or hindered neuron growth. Their work proves that HCS approaches can be useful for both drug development and basic research.
Finally, because it’s used to test a variety of outcomes, HCS can uncover surprising results when searching for therapeutics. For example, Quintavalle used High-Content Imaging to screen drugs and their effects on the invasive potential of cancer cells. [6] In addition to finding compounds that inhibited cells from metastasizing, they found that one known anti-tumor drug actually increased the invasive behavior of their cells.
In addition to the well-developed techniques that have been optimized for thoroughness and efficiency, the potential of HCS is continuing to be unlocked with new technological developments. For example, although CRISPR is not yet a widely used method in HCS, we are on our way to this being a reality. Recently, Yan et al. developed a high-content imaging approach to screen for genes that affect nuclear size regulation. [7] On the analysis end of the pipeline, informaticians are beginning to develop Deep Learning-based algorithms for High-Content Analysis, and have demonstrated their success in a screen to detect drugs with toxic side effects on cardiomyocytes. [8]
Finally, High-Content Screening has proven itself in its application to new and novel challenges, particularly recently. HCS systems have been co opted for the rapid and efficient isolation of the SARS-CoV-2 virus [9], and an HCS system has been developed that can be used to screen for drugs effective against COVID-19 infection in lung cells. [10]
Instruments and solutions for HCS
To streamline the HCS process, devices such as High Content Imagers exist that are specifically designed to analyze large numbers of samples in the exact same way. Oftentimes such devices can come packaged with their own software for High-Content Analysis.
High-Content Imaging devices are in many ways similar to the devices that researchers are already familiar with, being capable of widefield and confocal microscopy. The features that make these devices suited for screening include: the ability to image multi-well plates of various sizes, environmental controls (for maintaining temperature and CO2 levels in living cells), autofocus capabilities to better automate the imaging process, and a range of detectors to allow them to handle samples that have been stained with multiple dyes.
In addition, many systems have developed more sophisticated features specifically suited for HCI. For example, many High-Content Imagers are capable of automatically detecting objects in a well, tracking objects over time, drift compensation, and rare event detection.
If you’re in the market for a system to perform HCS, you have plenty of options. A brief sample of companies that make instruments and softwares for HCS include:
Read more about HCS on the web
- Photometrics: Introduction to High Content Imaging; Systems for High Content Imaging
- Drug Target Review: High-content imaging: challenges of the 3D world
- BioCompare: High-Content Imaging in 3D; Better Biology Drives HCS Advances
- Drug Discovery World: Latest Developments in High Content Screening Systems
References
- Starkuviene & Pepperkok. “The potential of high-content high-throughput microscopy in drug discovery.” British Journal of Pharmacology. (2007)
- Zhang et al. “Quantitative Phenotyping-Based In Vivo Chemical Screening in a Zebrafish Model of Leukemia Stem Cell Xenotransplantation.” Plos One. (2014)
- High Content Screening Trends 2016. HTStec Limited. (2016)
- Garripa et al. “High-throughput confocal microscopy for beta-arrestin-green fluorescent protein translocation G protein-coupled receptor assays using the Evotec Opera.” Methods in Enzymology. (2006)
- Blackmore et al. “High content screening of cortical neurons identifies novel regulators of axon growth.” Molecular and Cellular Neuroscience. (2010)
- Quintavalle et al. “A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion.” Science Signalling. (2011)
- Yan et al. “High-content imaging-based pooled CRISPR screens in mammalian cells.” Journal of Cell Biology. (2021)
- Grafton et al. “Deep Learning Predicts Patterns of Cardiotoxicity in a High-Content Screen Using Induced Pluripotent Stem Cell–Derived Cardiomyocytes.” BioRxiv. (2021)
- Francis et al. “High-speed large-scale automated isolation of SARS-CoV-2 from clinical samples using miniaturized co-culture coupled to high-content screening.” Clinical Microbiology and Infection. (2021)
- Marwick et al. “Application of a High-Content Screening Assay Utilizing Primary Human Lung Fibroblasts to Identify Antifibrotic Drugs for Rapid Repurposing in COVID-19 Patients.” SLAS Discovery. (2021)
- Lin et al. “Image-based high-content screening in drug discovery.” Drug Discovery Today. (2020)
- Fraietta & Gasparri. “The development of high-content screening (HCS) technology and its importance to drug discovery.” Expert Opinion on Drug Discovery. (2016)
- Zanella et al. “High content screening: seeing is believing.” Trends in Biotechnology. (2010)
- Markossian et al. “Assay Guidance Manual: Assay Development Guidelines for Image-Based High Content Screening, High Content Analysis, and High Content Imaging.” Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences. (2004–)